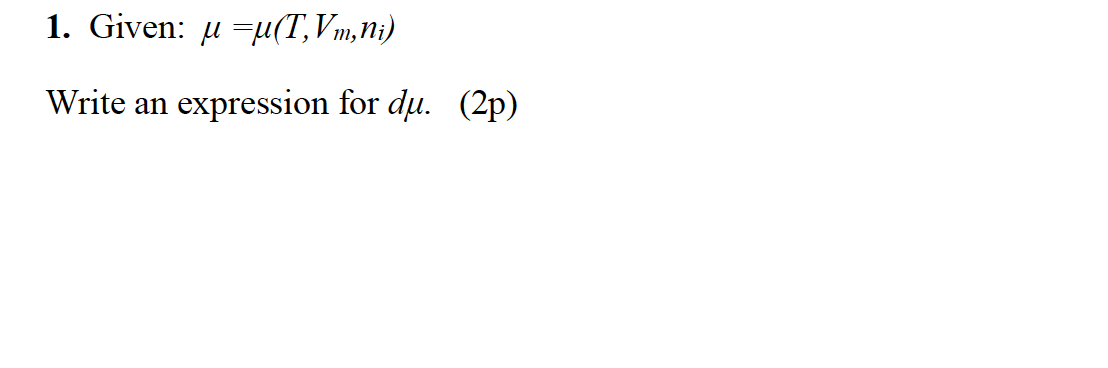![SOLVED: Fundamental constants and conversion factors: NA 6.022 *1023 mol-1 k (or kB) 71381*10-23] K-1 R=kNA 8.314 ] K-l mol-! = 8.314 Pa m3 mol-! E K-l 1.602 x10-19 C NAe = SOLVED: Fundamental constants and conversion factors: NA 6.022 *1023 mol-1 k (or kB) 71381*10-23] K-1 R=kNA 8.314 ] K-l mol-! = 8.314 Pa m3 mol-! E K-l 1.602 x10-19 C NAe =](https://cdn.numerade.com/ask_images/de3c0ad446614f81852d19db635e597b.jpg)
SOLVED: Fundamental constants and conversion factors: NA 6.022 *1023 mol-1 k (or kB) 71381*10-23] K-1 R=kNA 8.314 ] K-l mol-! = 8.314 Pa m3 mol-! E K-l 1.602 x10-19 C NAe =

Dose-dependent HASMC activation by Pam 3 CSK 4 , poly(I:C), LPS, R-837... | Download Scientific Diagram

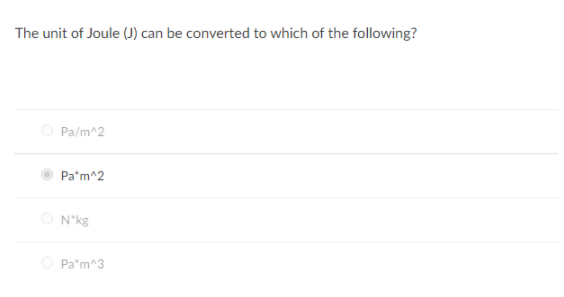
SOLVED: There are four additive terms in an equation, and their units are given below. Which one is not consistent with this equation? (a) J (b) W/m (c) kg·m2/s2 (d) Pa·m3 (e)

Temperature and thermal expansion Specific Heat Capacity Phase changes and Heat Molecular picture of a gas Ideal gas law Kinetic theory of. - ppt download
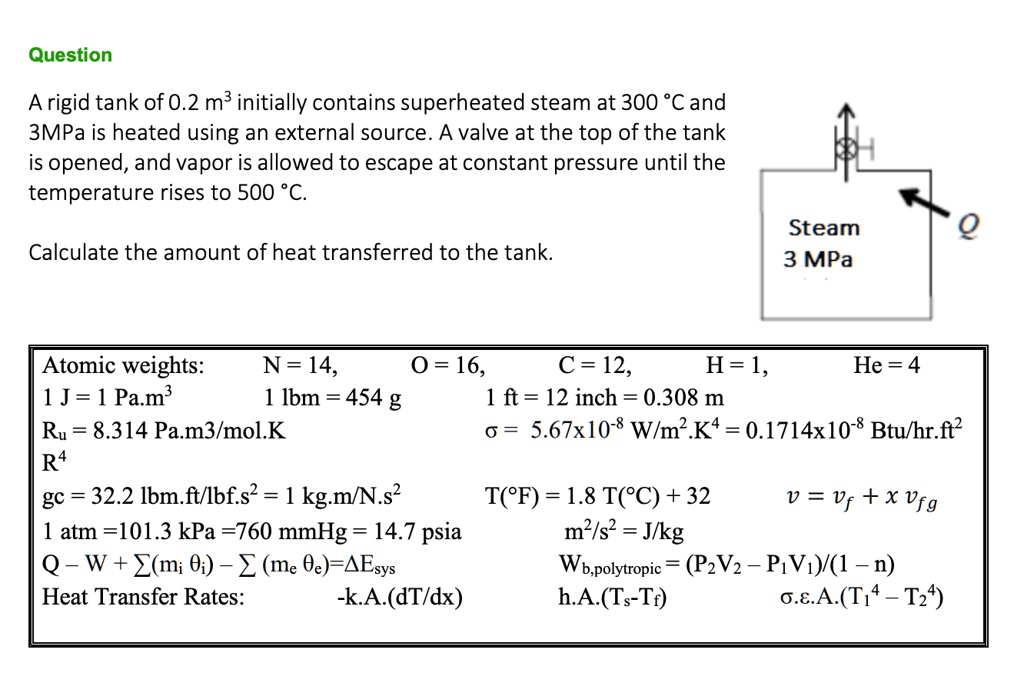
SOLVED: Question A rigid tank of 0.2 m3 initially contains superheated steam at 300 'C and 3MPa is heated using an external source. A valve at the top of the tank is
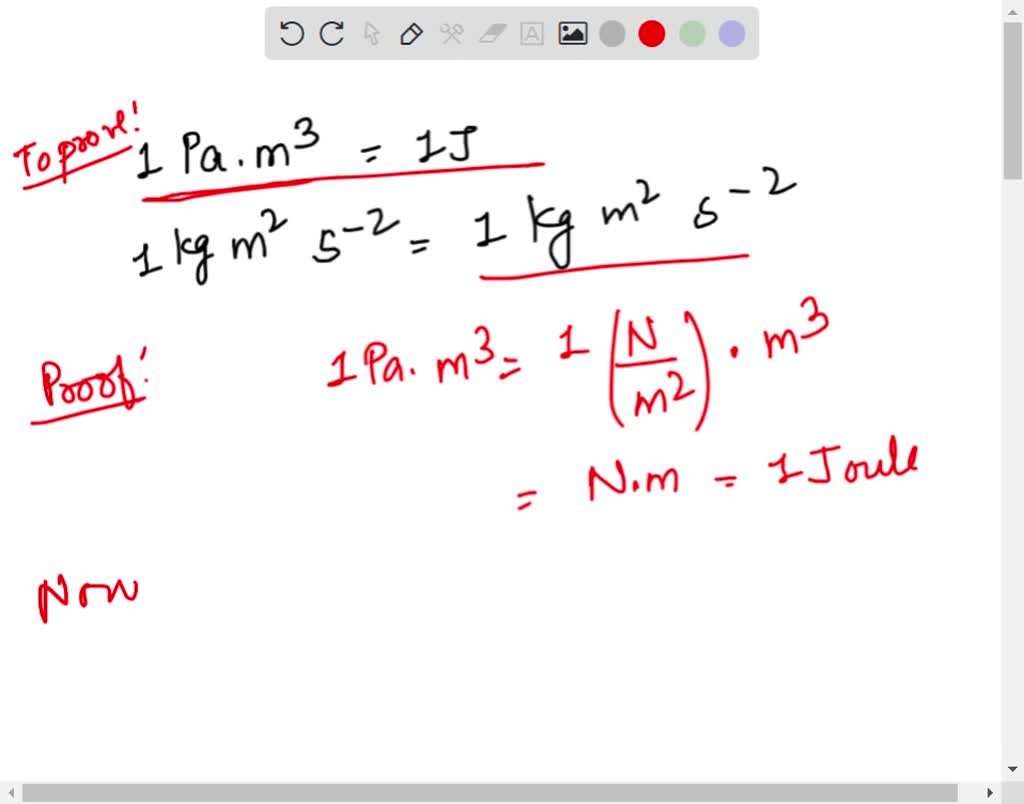
SOLVED: I just showed the conversion of 1Pa*m^3 to 1 J. My question is 1 kg*m^2/s^2 the same as 1kg*m^2/s^-2? Can you please give an explanation also?

Unit One Quiz Solutions and Unit Two Goals Mechanical Engineering 370 Thermodynamics Larry Caretto February 11, ppt download

Notes for CHEM1022, Chemistry for Pharmacy and Dentistry | CHEM1222 - Chemistry for Pharmacy and Dentistry - UQ | Thinkswap